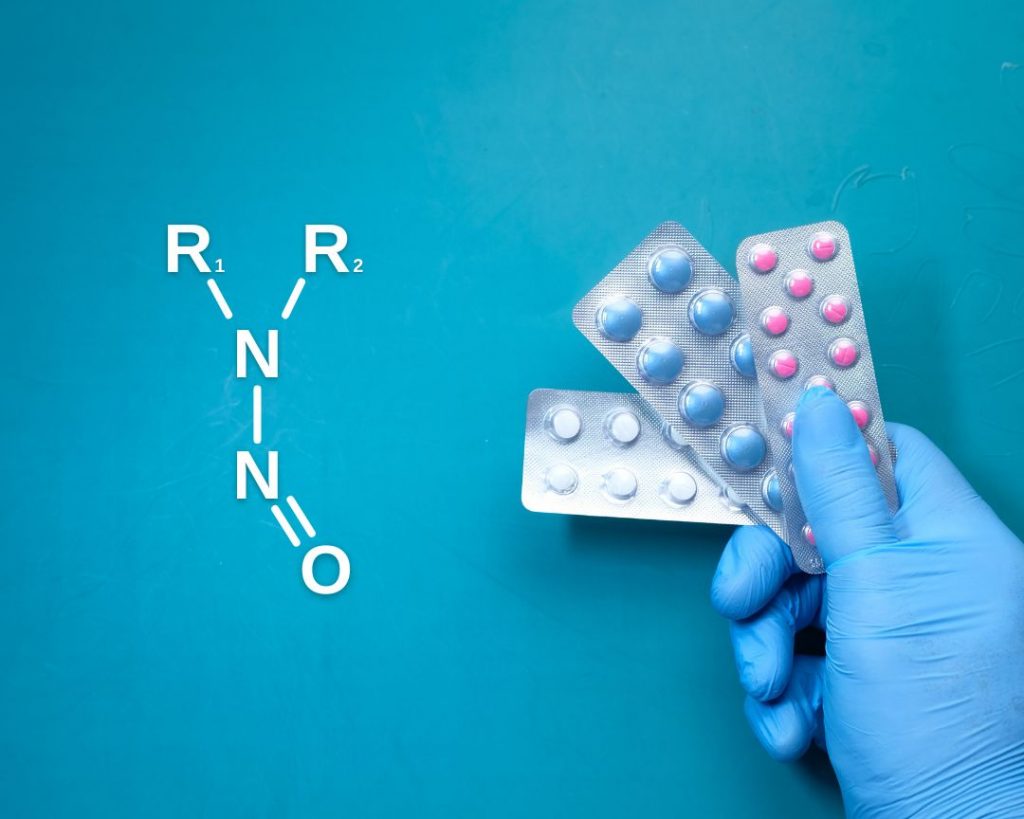
How to calculate the safe limit for carcinogens as per ICH M7
Mutagenic impurities can be expected in any drug product; however, this is not a concern providing they are present below the safe limit. The objective of a carcinogenicity study is to identify compounds that can cause tumours in animals, which is imperative to understanding the cancer risk posed to humans. ICH M7 in practice The […]
How to calculate the safe limit for carcinogens as per ICH M7 Read More »