Kaptis
Supporting accurate and cost-effective carcinogenicity risk assessment
- Determine the carcinogenic risk of your compound, using adverse outcome pathways (AOPs) to organise knowledge
- Reach a decision in your weight of evidence (WoE) assessment quickly, using an alternative approach for ICH S1 carcinogenicity assessments
- Lead the way in adopting the 3Rs, to reduce animal testing when it does not add value to the overall safety assessment
- Save up to $3.75 million in drug discovery costs and shorten development timelines by 2-3 years, bringing vital drugs to market faster
Moving towards non-animal new approach methodologies (NAMs)
Within many sectors of the life sciences industry, animal testing is still considered a primary option for toxicity testing, and as a result is used heavily. The limitations of this model are now well understood and non-animal new approach methodologies (NAMs) present an ideal opportunity to improve on the traditional approach.
But how do you overcome the challenges in validation, combining results and understanding NAMs?
By using adverse outcome pathways (AOPs) to organise this knowledge, the following questions can be answered:
Given this compound and/or this assay result, what adverse outcome (AO) is a cause for concern?
Given this predicted AO, what assay should I run next?

Kaptis uses AOPs to support you in answering these questions, to determine the carcinogenic potential of your compound
Our carcinogenicity AOP knowledge framework in Kaptis has been created, curated and peer reviewed by our expert Lhasa scientists. This framework and our Kaptis ICH S1 functionality, enables you to quickly reach a decision in your weight of evidence (WoE) assessment.
In some cases, it is possible to avoid running a full carcinogenicity animal study, where the WoE assessment clearly shows that it would add no value to the overall assessment.
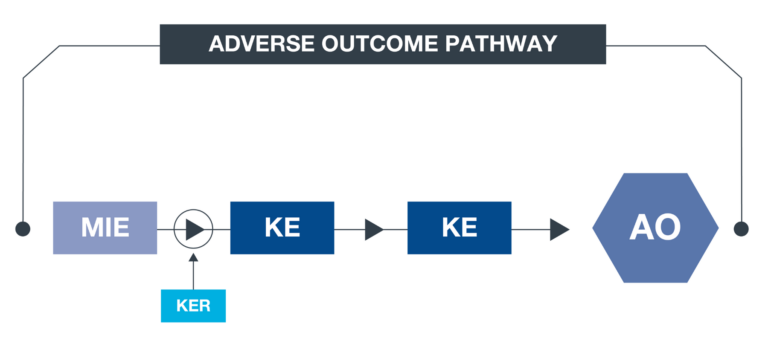
What is an AOP?
An AOP (adverse outcome pathway) is an approach to documenting causal relationships between biological processes which lead to adverse outcomes/ toxicity. AOPs start with a molecular initiating event (MIE) and through additional key events (KEs), lead to an adverse outcome (AO). Each sequential KE (including the MIE and AO) are connected to each other through key event relationships (KERs). Each KE should be measurable and therefore can be linked to a relevant assay.
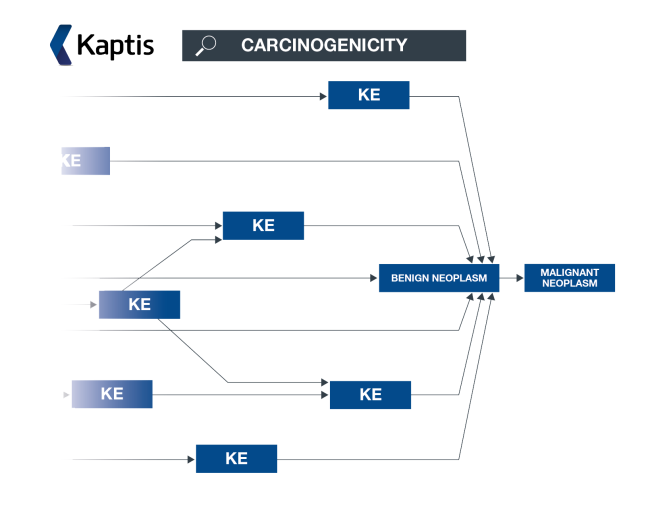
What is an AOP network?
An AOP network allows interconnecting KEs across different AOPs to be easily investigated as a network.
Regulatory support
ICH S1
The addendum within the ICH S1B(R1) pharmaceutical guideline addendum states, if a weight of evidence (WoE) assessment clearly shows that there is no value to the overall pharmaceutical assessment in running a full carcinogenicity animal study, then this evidence can be submitted as alternative. Kaptis can be used to address this WoE approach, including considering the 6 factors outlined in the addendum which account for different mechanisms that can lead to cancer.



Explore our other in silico solutions that can support you in your carcinogenicity risk assessments; the Lhasa Carcinogenicity Database (LCDB), Vitic, and Derek Nexus.
Related publications
Paper
- Mar 2025
- Safety profiling in drug discovery
Paper
- Aug 2024
- Safety profiling in drug discovery
Poster
- Mar 2024
- Safety profiling in drug discovery