Carcinogenicity assessment
Supporting accurate and cost-effective
carcinogenicity risk assessment
Across the life sciences industry and beyond, there is a notable and conscious shift away from long term carcinogenicity animal studies. The objective of a carcinogenicity animal study is to identify compounds that can cause tumours in animals, and thereby increase the understanding of the cancer risk posed to humans. Within many sectors of the life sciences industry, animal testing is still considered a primary option for toxicity testing, and as a result is used heavily – especially within the pharmaceutical sector. However, the limitations of this model are now well understood and non-animal new approach methodologies (NAMs) present an ideal opportunity to improve on this current approach and provide alternative approaches to assessing carcinogenic risk.

The Lhasa Carcinogenicity Database (LCDB) is a significant source of long-term carcinogenicity studies and can help you to understand if there is existing data available for your compound.
LCDB highlights
Access actively maintained public carcinogenicity data for free
The data within the LCDB is presented within a user friendly and standardised interface. We continuously update the database, as new data becomes available. The LCDB was founded in 2016 on the now retired Carcinogenic Potency Database (CPDB), created by Lois Gold and her team. The database is free for everyone to access.
View TD50 values which can help you to calculate acceptable intakes
TD50 values are an important aspect of any carcinogenicty assessment. TD50 values indicate the lifetime dose at which tumours would be observed in 50% of animals, for a given compound, which otherwise would have remained tumour free without dosing. There are two TD50 values in the LCDB; Lhasa TD50 and CPDB TD50. We have calculated the Lhasa TD50 values based on the values provided by Lois Gold and her team, producing a transparent methodology for calculating TD50 values from experimental data, in a manner consistent with the CPDB. Displaying both TD50 values in the LCDB has enabled validation of the calculation method by comparison with the original CPDB values. As we expand the LCDB data set, we now also calculate TD50 values for the newly added data.
Find similar analogues using substructure or similarity searches
Where there is not an exact match for your compound in the LCDB, you can make use of the substructure or similarity search functionality – which can be used to help define the acceptable limit.

Vitic is a source of high-quality, peer-reviewed public and proprietary carcinogenicity data. When used alongside the LCDB, Vitic can help you to understand if there is existing data available for your compound.
Vitic highlights
Access high-quality public and proprietary data
The data within Vitic is expert-curated, high-quality, peer-reviewed data from both published and unpublished sources.
Search for data within other related endpoints
Alongside carcinogenicity data, you can also search for data within other related endpoints such as mutagenicity and chromosome damage, which can help to build your understanding of a carcinogenic risk.
Find similar structural analogues using substructure or similarity searches
Where there is no exact match within Vitic, you can make use of the substructure or similarity search functionality.

Derek Nexus is an expert, knowledge-based prediction software that can help you to establish if there is a carcinogenicity hazard associated with your compound, in cases where there is no existing data available.
Derek Nexus highlights
Access over 40 years of expert Structure-Activity Relationship (SAR) knowledge for the carcinogenicity endpoint
Derek contains well-established Structure-Activity Relationship (SAR) knowledge for the carcinogenicity endpoint. The SAR knowledge is derived from both public and proprietary member data sources and is updated as new data becomes available.
Identify any toxicophores highlighting possible carcinogenic hazard
Assess your compounds against key toxicity endpoints in relation to expert derived toxicophores. The expert comments associated with a Derek Nexus alert can help you to decide if this compound structure is a concern or not.
Understand the SAR behind carcinogenicity predictions
The scientific comments which accompany a Derek Nexus alert will describe the alert mechanism of action.

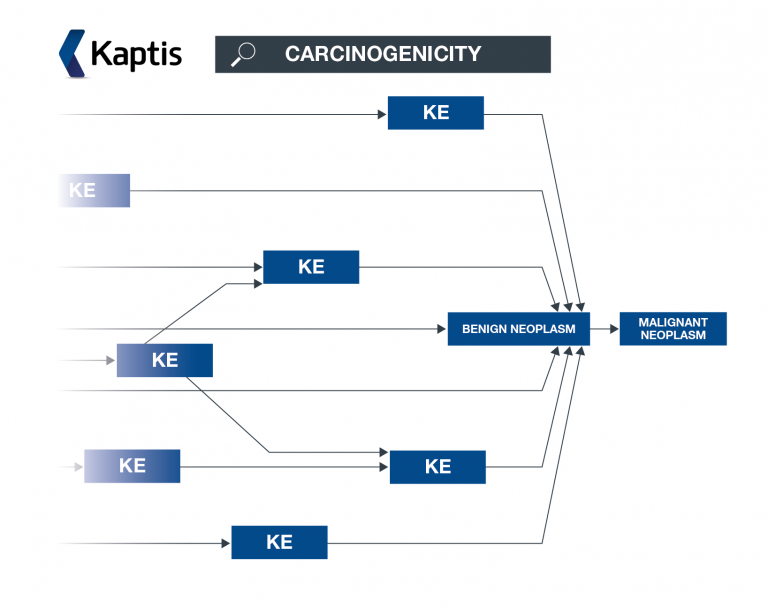
Kaptis is a novel decision support solution that helps determine the carcinogenic potential of a compound, using adverse outcome pathways (AOPs) to organise knowledge in line with best practice.
Kaptis highlights
Explore relevant carcinogenicity knowledge
You will be able to access carcinogenicity AOPs and associated knowledge within our extensive AOP carcinogenicity knowledge framework which has been created, curated and peer reviewed by our expert Lhasa scientists. You will also be able to view AOPs within an AOP network – helping you to understand the broader impact of a key event.
View an overall ICH S1 classification prediction for your compound
Quickly reach a decision in your weight of evidence (WoE) assessment, using our Kaptis ICH S1 functionality. In some cases, it is possible to avoid running a full carcinogenicity animal study, where the WoE assessment clearly shows that there is no value to the overall assessment in running a full animal study.
Streamline any further testing required
By answering questions such as; “Given this compound and/or this assay result (irrespective of the chemical), what adverse outcome (AO) should I worry about?” or “Given this AO, what assay should I run?” you can improve the efficiency of any further testing required. Resulting in more informed, faster, and more cost-effective human safety assessment decisions.
Regulatory support
ICH S1
The addendum within the ICH S1B(R1) pharmaceutical guideline addendum states, if a weight of evidence (WoE) assessment clearly shows that there is no value to the overall pharmaceutical assessment in running a full carcinogenicity animal study, then this evidence can be submitted as alternative. Kaptis can be used to address this WoE approach, including considering the 6 factors outlined in the addendum which account for different mechanisms that can lead to cancer.
ICH M7
The ICH M7 guideline is designed to establish the carcinogenic risk posed by mutagenic impurities, considering both safety and quality risk management. If a compound reacts with DNA it may lead to a mutation, which is the first step in the development of cancer. This can be a risk even for low level impurities. The guideline states that the availability of carcinogenicity data must be taken into consideration, the availability of carcinogenicity data affects the class which will be assigned to the impurity in order to determine the next steps.
REACH Regulation (EC) No. 1907/2006
This regulation from ECHA relates to chemicals which are produced, imported or used in large quantities. The information requirements depend on quantity of the chemical produced, imported or used. If the threshold for carcinogenicity information is triggered, we have a carcinogenicity QMRF document available for the use of Derek this purpose – as recommended within the guidance on information requirements and chemical safety assessment.
Supporting information
What is an AOP?
An approach to documenting causal relationships between biological processes which lead to adverse outcomes/ toxicity. AOPs start with a molecular initiating event (MIE) and through additional key events (KEs), lead to an adverse outcome (AO). Each sequential KE (including the MIE and AO) are connected to each other through key event relationships (KERs). Each KE should be measurable and therefore can be linked to a relevant assay.
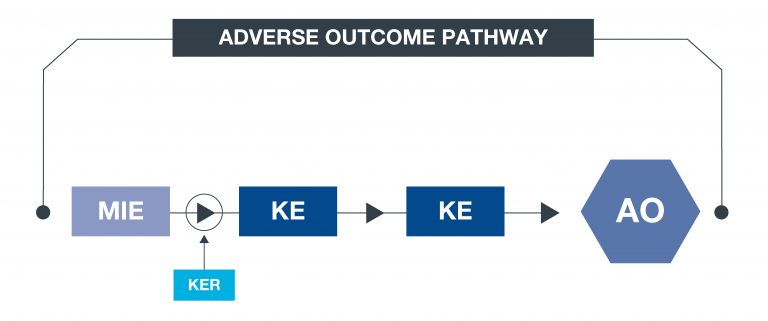
What is an AOP network?
An AOP network allows interconnecting KEs across different AOPs to be easily investigated as a network.
Related publications
Paper
- Feb 2026
- Carcinogenicity assessment, Nitrosamine impurity risk assessment
Poster
- Aug 2025
- Carcinogenicity assessment, Safety profiling in drug discovery
Paper
- Jul 2025
- Carcinogenicity assessment
Recent blogs
Mutagenicity assessment is no longer debated; it is embedded within regulatory science. The more important question today is not whether in silico …
Approaching regulatory compliance with confidence ICH Q3D guidelines require assessing and controlling elemental impurities for regulatory compliant development of drug products. The …
We’re delighted to share that Lhasa Limited has been shortlisted for the Lush Prize 2026 in the Science category, an award recognising …