Introducing new and updated AI/PDE shared values to strengthen consistency in regulatory submissions detailing acceptable exposure limits.
Lhasa Limited is pleased to share an expanded database for acceptable intake (AIs) and permitted daily exposure (PDE) limits, now available to new and existing members of the Vitic AI/PDE data sharing initiative.
This release is another milestone in the journey towards shared knowledge and shared scientific progress to better human health and rule out unnecessary animal testing.
Vitic AI/PDE is an exclusive data sharing initiative that harmonises solitary AI/PDE data to streamline values across industry ahead of regulatory submission. Improving both the time taken to submit and the consistency in documentation.
Discover a portfolio of peer-reviewed monographs from world-leading experts
The precompetitive collaboration facilitates the sharing of AI/PDE limits to reinforce consistent AI/PDE values for the same chemical when being submitted to regulators.
The AI/PDE 2026.1 release from the Lhasa data sharing initiative expands the database to include:
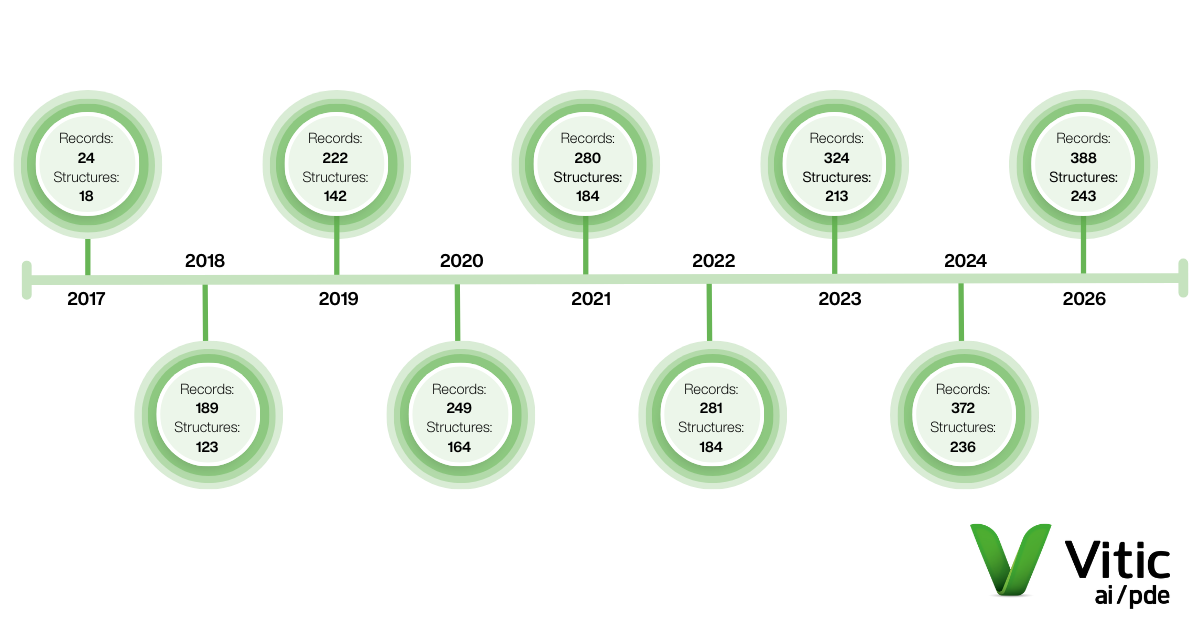
- 16 new data records, bringing the total to 388.
- 7 new substances, bringing the total to 243.
- AI/PDE values for over 240 common pharmaceutical intermediates.
- 31 updated PDE records to align with current monograph generation across the sector.
What does this mean for industry?
Participation in the initiative sees key organisations contribute towards the continued growth of the AI/PDE database, as well as produce harmonised datasets to utilise for decision-making on common impurities.
Access to the latest records in Vitic AI/PDE requires membership in the Vitic AI/PDE consortium and the ability to participate in monograph generation and peer review activities.
Shared data, shared knowledge, shared progress
Vitic AI/PDE welcomes its latest cycle of culminated high-quality monographs, a dataset that has continued to grow since its establishment/curation in 2017.
Recognising the tendencies for organisations to develop compound-specific AI/PDE limits independently, the data sharing initiative and its consortium continue to align their conclusions and methodologies. Each year, the AI/PDE database is expanded, encouraging industry to work in partnership, not silos.
By redirecting time spent on extensive literature review from potentially varying AI/PDE values towards precompetitive data review, organisations can streamline their workflows whilst positively impacting communal developments in safety decision-making.
Each member of the data sharing initiative (including a vast majority of the top 20 pharmaceutical organisations globally) has collaborated to produce precompetitive transparency across AI/PDE values, in order to:
- Save time in AI/PDE risk assessments through shared resources and collective monograph production across industry.
- Reduce duplication in AI/PDE data by quick access to a structure-searchable AI/PDE database.
- Improve standardisation through collaborative curation and continuous update of shared monographs.
- Streamline regulatory submission consistency by utilising agreed AI/PDE data for ICH M7, ICH Q3C, and ICH Q3D, amongst other reports.
The creation of harmonised monographs for common impurities has proven to save the time and resources of our members, all while safeguarding human health.
Participate and contribute towards the most robust data
Data sharing allows industry to approach their risk assessment workflows with confidence, by utilising data that has been contributed and peer-reviewed to reach its height of accuracy.
Newly added data records are vigorously evaluated before being added to the AI/PDE dataset. This tackles consistency and reliability challenges directly.
With easy methods to share and corroborate data, at a precompetitive development stage, we can facilitate shared understanding, growth, and impact without disclosure of sensitive internal information.
The AI/PDE data sharing 2026.1 release is a result of a consortium motivated by consistency in regulatory submissions.
This is just one example of how data sharing initiatives at Lhasa can benefit your own toxicity endpoint of relevance, whilst helping to identify single sources of truth for the wider pharmaceutical community.
By joining, new members will unlock 388 total AI/PDE records across 243 substances, including the very latest PDE monograph records.
