Introducing Vitic Aromatic Amines 2025.1.0, featuring newly shared data from Ames mutagenicity assays, a result of over a decade of pre-competitive pharmaceutical collaboration.
Lhasa Limited is pleased to share an expanded primary aromatic amines (PAAs) dataset, now available to benefit both shared knowledge and shared scientific progression in the mechanistic understanding of PAAs.
The Vitic Aromatic Amines 2025.1.0 release is an exciting development in the data sharing initiatives from Lhasa Limited.
The knowledge from data initiatives are used to fuel in silico prediction tools to benefit chemical safety decision-making and, in turn, human health. By enabling access to public and proprietary Ames data, market-leading organisations can work together to advance their understanding of Ames mutagenicity for PAAs.
Introducing the Primary Aromatic Amines Data Sharing Initiative
The Consortium for the Investigation of Genotoxicity of Aromatic Amines (CIGAA) is a pharma-wide approach to refine current understanding of structure-activity relationships (SARs) and resulting biological effects.
Formed to tackle the challenge of confident prediction of aromatic amine mutagenic activity, the consortium prioritises data sharing to instill knowledge across companies and clarify the underlying mechanisms of mutagenic activity.
With this, the latest Vitic Aromatic Amines 2025.1 release also accounts for substantial additional genetic toxicity in vitro data, resulting in 93% of all data records in the set occupying DNA damage or mutagenicity insights.
Discover the latest consortium-derived data in Vitic Aromatic Amines
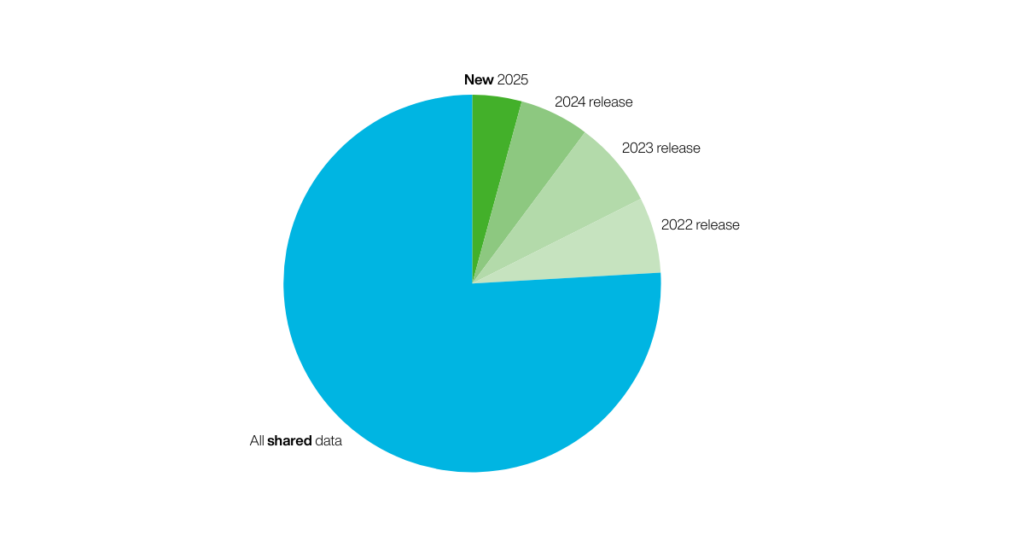
The latest release encompasses 833 new data records and 49 new substance structures.
This results in our dataset now containing 21,400+ data records in total across 1,300+ structures.
What does this mean in practice? Here are some key areas of the latest release which highlight safer and more efficient workflows in pharmaceutical development:
- Expanded coverage of data reduces testing requirements and duplication risks
- Strengthened mechanistic understanding for expert deployment in genotoxic risk assessment
- Improved predictability of PAA alerts within Derek Nexus to identify safer building blocks in the drug synthesis planning process
- Collectively, all three benefit the wider scientific community through a rich knowledge base, which brings confidence to decision-making.
To access the latest records in Vitic Aromatic Amines, you must be a member of the Vitic solution and participant in the consortium.
With new contributions made every year, the database continues to strengthen in scientific value. Expanding annually, Vitic Aromatic Amines 2025.1.0, represents the commitment of industry-leading pharmaceutical organisations to advance knowledge and innovate safety in drug development.
Making decisions more confident and predictions more reliable:
Donate data and play a role in industry progression
Siloed data means only test results for the compounds an organisation has worked on are available. From here, pattern identification and reliability in prediction for genotoxicity of new compounds can delay discovery and development of new pharmaceutical products.
Data sharing helps industry approach genotoxicity from a wider landscape of knowledge to tackle reliability challenges head-on. The anonymously shared data, at a pre-competitive development stage, can result in more informed decisions on safety.
A larger set of real-world experimental results helps organisations learn from one another, collaboratively progressing efforts to positively impact human health.
Vitic Aromatic Amines 2025.1.0 is a result of rigorous data collected from various leading pharmaceutical projects, with Lhasa data sharing initiatives also spanning other aspects of genotoxicity risk assessment.
Joining the consortium
Ready to share and access proprietary Ames data of PAAs? Get in touch with Lhasa to contribute toward improving the reliability of in silico predictions, save time and improve efficiency in your own workflow simultaneously.
By joining, members will also uncover the 833 new data records and 49 new substance structures available in Vitic Aromatic Amines 2025.1.0.
