One of our three Lhasa Limited posters during the recent Society of Toxicology 63rd annual meeting was selected for the top 10 best risk assessment abstract award by the Risk Assessment Speciality Section!
Authored by Lhasa scientist, Pooja Tomar, the poster titled “Best practice for conducting expert review of sensitisation data and predictions” highlights the critical importance of implementing best practices in skin sensitisation risk assessment.
Skin sensitisation, a crucial toxicity endpoint across various industries including cosmetics, industrial chemicals and pharmaceuticals (in relation to extractables and leachables), demands meticulous evaluation for accurate potency assessment. Pooja’s poster highlights the significance of expert review in interpreting sensitisation data and predictions, enhancing assessment confidence, and resolving conflicts. By establishing key principles and best practices, the poster aims to ensure consistent application of methods and reasoning for regulatory acceptance.
Understanding RASS
Risk Assessment Speciality Section (RASS) within the Society of Toxicology (SOT) serves as a platform for the exchange of information, the advancement of scientific discussions and the recognition of excellence in risk assessment methodologies. Through awarding outstanding scientific contributions, the RASS fosters collaboration, innovation and progress in the field of toxicology.
A panel of volunteers reviewed and evaluated the initial pool of 283 risk assessment abstracts, ensuring impartiality by conducting blind assessments across four key categories:
- Application to risk assessment
- Scientific significance of the research/project focus
- Quality of the abstract
- Significance of the findings described in the abstract.
Expressing her gratitude, Pooja Tomar said “It’s an incredible feeling to be recognised among the top 10 abstracts in the risk assessment category at SOT. This nomination stands as a testament to the collective effort and dedication of everyone who contributed to this poster. I extend my gratitude to Martyn Chilton, Adrian Fowkes and Charles Modlin for their contributions and unwavering support.”
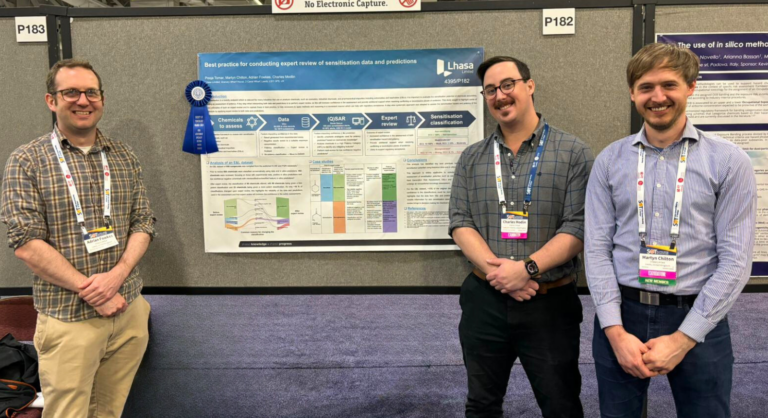
Pictured from left to right are poster co-authors; Head of Science, Adrian Fowkes, Senior Application Scientist, Charles Modlin and Principal Scientist, Martyn Chilton. In Pooja’s absence, the poster was presented by co-author, Charles Modlin at the 63rd annual meeting in Salt Lake City, Utah.
Read the poster to discover…
- A framework for conducting a toxicological evaluation of extractables and leachables (E&Ls) based on historical data and in silico predictions (for example, within the context of the upcoming ICH Q3E guideline).
- An approach that is widely applicable to substances which require an assessment of sensitisation potential, such as cosmetic ingredients within a Next Generation Risk Assessment, E&Ls and chemicals which need to undergo an occupational toxicology assessment.
- How our software solutions for high-quality, peer-reviewed data, Vitic and in silico toxicity prediction, Derek Nexus can provide reliable information for skin sensitisation assessments and how expert review can increase confidence in safety assessments.
The shortlisted poster is available for further exploration on our publications page, offering insights into these cutting-edge methodologies that are shaping the future of risk assessment.
Visit our skin sensitisation assessment page to discover in silico solutions to support your skin sensitisation risk assessment.
Last Updated on October 17, 2024 by lhasalimited