Carcinogenicity assessment is a critical step in drug development. Teams must balance regulatory compliance, development timelines, and ethical testing, often with incomplete datasets.
The revised ICH S1B(R1) guideline introduces a weight-of-evidence (WoE) approach that provides a more structured, efficient, and human-relevant path forward.
By applying this framework, organisations can benefit from:
- $2 – 4M cost savings per compound by avoiding unnecessary studies
- Up to 5 years shorter timelines, accelerating promising therapies to patients
- Reduced animal use, with a shift toward human-relevant evidence
- Stronger alignment with regulators, reducing uncertainty and risk.
But is it always easy knowing where to begin?
In this guide, we outline a five-step process to applying ICH S1B(R1) effectively, and show how our in silico decision support system, Kaptis, can support confident carcinogenicity risk assessment at every stage.
Step 1 – Understand the guidance
The ICH S1B(R1) addendum sets out how to assess the carcinogenic potential of pharmaceuticals using a WoE approach. At its heart, it asks:
Would a traditional two-year rat study add real value for understanding human risk?
The first step is about gaining clarity. Understanding the expectations of regulators, and how industry is adapting, provides the foundation for confident decisions. Kaptis helps at this stage by grounding your data in an expert-curated adverse outcome pathway (AOP) framework, so from the outset you can clearly see how evidence aligns with biological plausibility.
To help navigate this guidance, we hosted a webinar alongside ex-ICH S1B industry and regulatory expert working group representatives:
ICH S1B(R1) industry and regulatory best practice for confident carcinogenicity assessment, where experts shared how regulators and industry are applying these principles in practice.
Step 2 – Gather relevant data
ICH S1B(R1) highlights six key factors to consider:
- Target biology
- Secondary pharmacology
- Histopathology from chronic studies
- Hormonal effects
- Genotoxicity
- Immune modulation
Bringing these datasets together can feel overwhelming, as the evidence comes from diverse in vitro, in vivo and in silico sources.
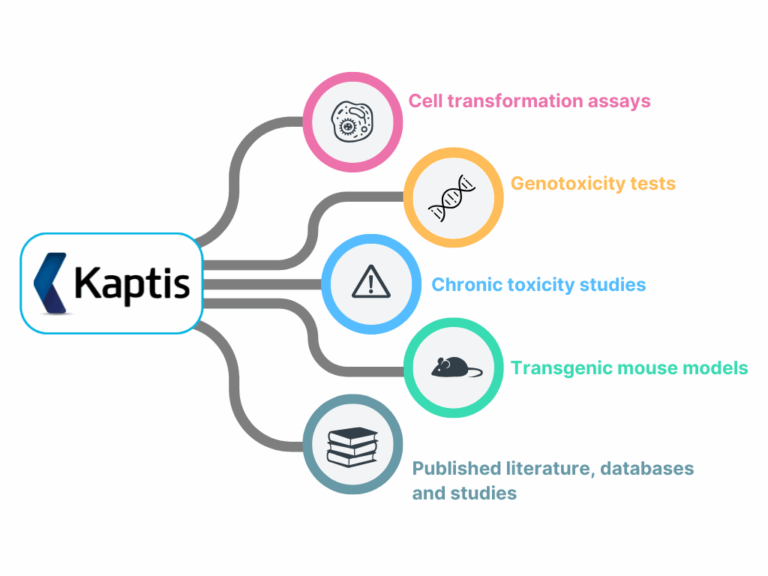
With the right structure, these diverse datasets can be brought together into a clear, connected picture, helping you to identify risks sooner and with greater confidence. Kaptis simplifies this step by providing an expert-derived AOP framework that maps evidence across all 6 factors into a visual network. This creates a common language to explore cause-and-effect relationships that underpin carcinogenic outcomes, helping you identify risks sooner and with greater confidence.
Step 3 – Apply Weight of Evidence
Here’s where judgment matters most. The WoE approach isn’t about ticking boxes; it’s about interpretation. Which findings are most relevant? How reliable is the data? Where does the evidence converge, and where does it diverge?
Kaptis supports expert review with a question-based approach that guides your interpretation. This ensures your WoE is not only scientifically robust but also transparent and regulator-ready, giving your teams confidence in their rationale.
Step 4 – Use a decision support system
Integrating diverse datasets is complex, and the process can feel daunting. Decision support systems like Kaptis can make a real difference.
Kaptis doesn’t replace scientific judgment, it enhances it. By providing structure, consistency, and transparency, Kaptis allows your teams to focus on what matters: confident, well-supported conclusions.
Organisations that take this approach often find it reduces uncertainty, saves valuable time, and helps them feel more prepared for regulatory submissions.
Step 5 – Document the conclusion using best practice
Finally, it’s about sharing your thinking. Regulators want to see your data, and also the reasoning behind your decisions. Documenting your WoE assessment clearly and transparently builds trust and helps reviewers follow your logic. Kaptis automatically generates structured reports, supporting clear, reproducible documentation. This makes regulatory interactions smoother, while ensuring internal consistency across projects.
At our upcoming webinar, we’ll explore a case study that shows this approach in practice – so you can see what good looks like.
Catch up on our webinar to discover ICH S1B(R1) industry and regulatory best practice for confident carcinogenicity assessment.
Discover how Kaptis can simplify your carcinogenicity assessments.
Last Updated on January 9, 2026 by lhasalimited