Are you interested in collaboratively using data to predict and avoid adverse drug reactions? The Effiris consortium – currently composed of Takeda, GSK and UCB – is working to achieve just that.
The project aims to help pharmaceutical organisations accelerate drug discovery through machine learning.
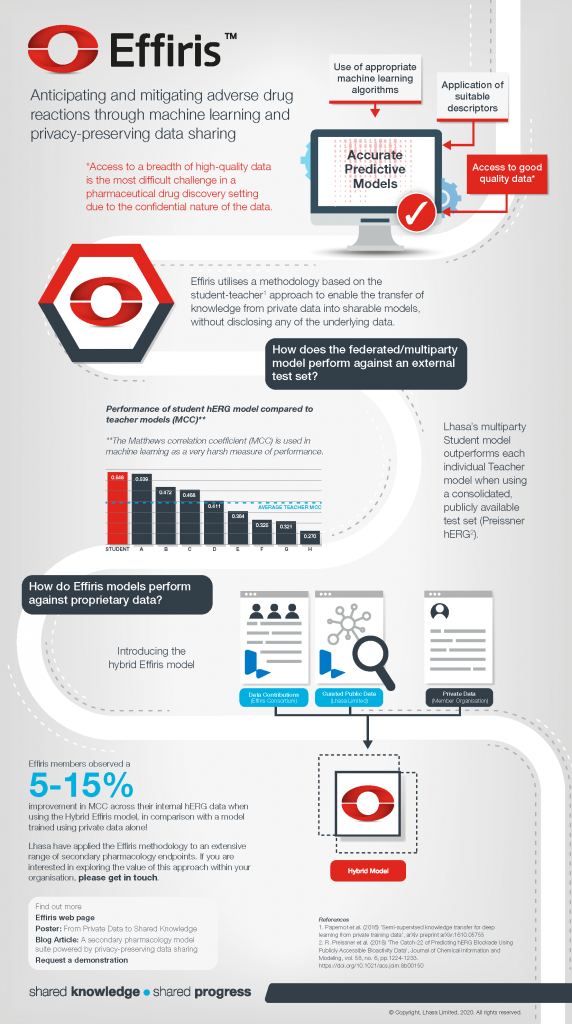
Many readers will be very familiar with the fact that one of the biggest challenges in building accurate predictive models within the pharmaceutical drug discovery space, is the limited availability of high-quality data, due to the often-confidential nature of the data. The Effiris privacy preserving data sharing methodology aims to overcome this obstacle.
Read more about the Effiris approach and progress in the infographic below.
________________
Did you enjoy this blog? Please let us know.
What else would you like us to write about? Please let us know.
Last Updated on January 25, 2024 by lhasalimited