Insights on ICH S1B(R1), genotoxicity and best practices in toxicology.
The 59th Congress of the European Societies of Toxicology (EUROTOX) brought the global toxicology community together in Athens under the theme “Toxicology addresses society’s real-life risks for sustainable health and well-being.” Discussions throughout the congress reflected a shared ambition: applying science and innovation to deliver safer medicines, chemicals, and environments while advancing sustainability.
Lhasa Limited in action
Lhasa was proud to contribute across multiple sessions at EUROTOX. On the first day of the conference, we hosted a well-attended industry session on genotoxicity, which explored regulatory expectations and shared real-world case studies on applying in silico tools in genotoxicity assessments.





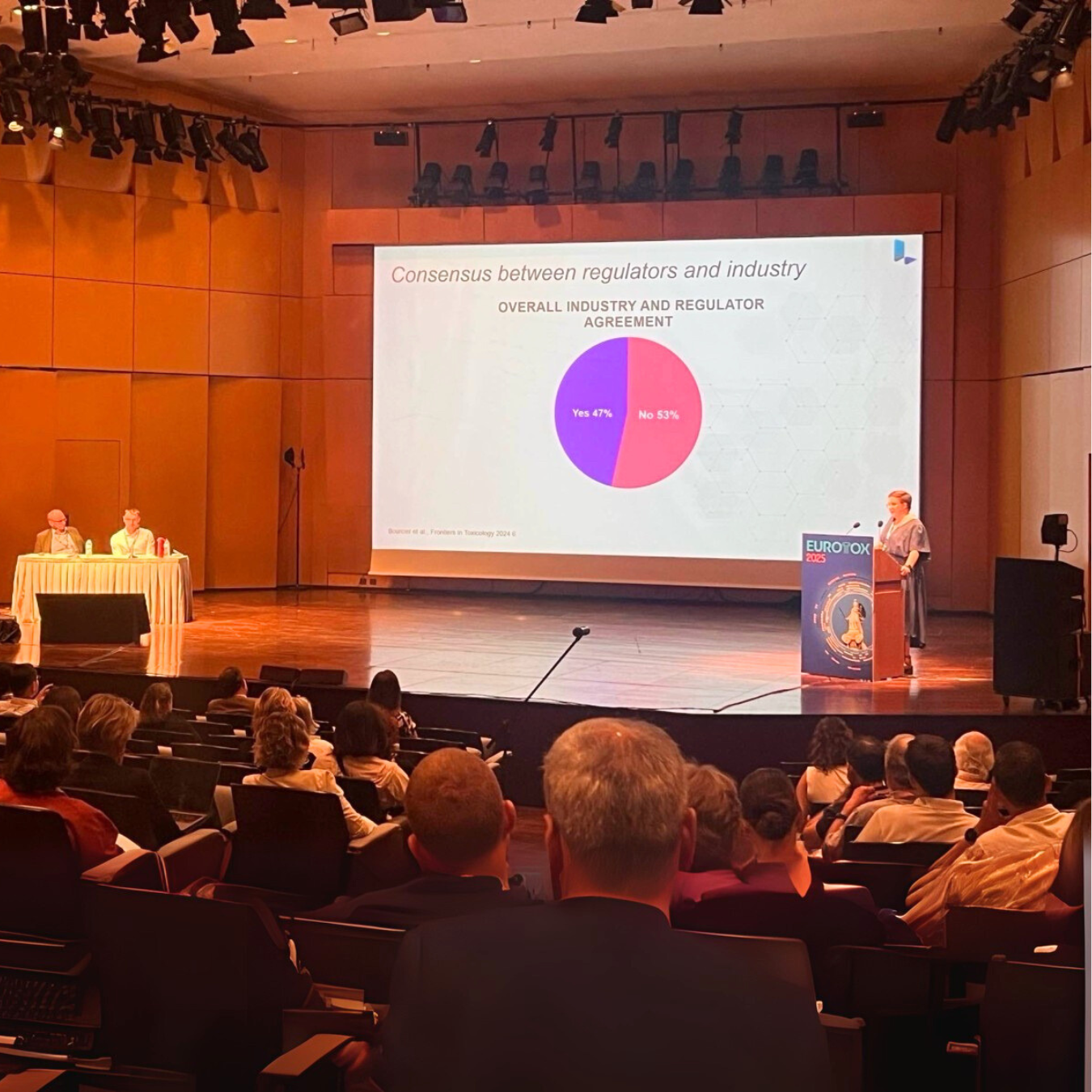

We also contributed to the scientific session “Carcinogenicity testing after the ICH S1B revision – where are we now?” chaired by Richard Knight (ApconiX, UK) and Paul Baldrick (Fortrea, UK). Lhasa Principal Scientist Dr Susanne Stalford closed the session with a presentation on how in silico decision support systems are shaping next-generation carcinogenicity risk assessment. The talk highlighted how computational methods can support regulatory decision-making, delivering consistent, scientifically robust predictions that reduce reliance on animal testing while helping scientists make confident safety assessments.
Key insights: Carcinogenicity testing after the ICH S1B(R1)
The session surfaced perspectives from regulators, industry, and study design experts, illustrating both the promise and challenges of ICH S1B(R1) implementation:
- Regulatory harmonisation – Kris Siezen (Medicines Evaluation Board, NL) reported that weight-of-evidence (WoE) assessments have already spared more than 20,000 animals to date. However, differences in interpretation of evidence across regions persist. A dedicated ICH S1 working group is now focused on harmonising approaches globally.
- Industry experience – John Vahle (Lilly, USA) emphasised the benefits of WoE assessments, including integrated assessments, leverage of investigative approaches to support reports and reduced animal use. Yet challenges remain with assessment timing, review variability across agencies, and impacts on development timelines.
- Study design – Paul Baldrick (Fortrea, UK) underlined the importance of optimised study design when testing novel small molecule entities for carcinogenicity in animals, showing how improvements can both strengthen outcomes and reduce animal use. Further information on this study can be found in the publication Carcinogenicity testing in drug development: Getting it right.
A common theme emerged: the need for consistency, clarity, and best practice in applying ICH S1B(R1). In silico decision support systems such as Kaptis, can directly address this need, reduce ambiguity and provide the transparency required for confident regulatory and scientific decision-making.
Continuing the conversation
To build on these discussions, Lhasa is hosting a webinar:
ICH S1B(R1) industry and regulatory best practice for confident carcinogenicity assessment.
We will bring together former ICH S1B working group experts from both regulatory and industry backgrounds to share how the guidance is being applied in practice and what the future potentially holds. It offers a unique opportunity for scientists, regulators, and industry professionals to gain practical insights into confident carcinogenicity assessment.
Standout sessions and emerging trends
Standout session – Beyond the first hype: real-life applications of artificial intelligence in contemporary toxicology and risk assessment.
Artificial intelligence (AI) is becoming part of everyday life, and toxicology is no exception. This session showcased the practical applications of AI in risk assessment, including machine learning based on 3D molecular structures to improve toxicity prediction, the use of gene databases to forecast drug-induced cholestasis hazards, and text mining combined with graph theory to advance the development of adverse outcome pathways.
Emerging trend – Use of New Approach Methodologies (NAMs) and modelling to assess toxicity in next generation risk assessment (NGRA).
A recurring theme throughout EUROTOX 2025 was the growing role of NAMs in toxicology. Beyond their established use in WoE assessments for carcinogenicity, NAMs are now being applied to cardiotoxicity and immunotoxicity. In the session “Cardiotoxicity: A roadmap to regulatory acceptance”, speakers outlined how NAMs could provide mechanistic, human-relevant data that may eventually replace animal studies in WoE assessments. Similarly, the session “NAM in Immunotoxicology: from in vitro to risk assessment” highlighted advances for both small molecules and biologics.
Perhaps the most forward-looking discussion came from “The Virtual Human in Toxicology”, where Steve Levine (Dassault Systèmes, US) delivered a powerful talk on how virtual twins, digital replicas of human biology, played a role in saving both his daughter’s life, as well as his own. Collectively, these sessions reflected a clear momentum: scientists are eager to move away from animal testing and toward approaches that are both ethical and more predictive of human outcomes.
Thank you to everyone who visited us at our booth and attended our industry-hosted session. We look forward to staying connected and continuing the conversation!
If you would like a personalised demonstration of our in silico solutions, you can schedule a meeting with our business partnerships team.
Last Updated on September 30, 2025 by lhasalimited