Introduction to Lhasa Carcinogenicity Database
For many years the Carcinogenic Potency Database (CPDB)1, created by Lois Gold and her team, was an important source of long-term carcinogenicity study data. However, as the database had stopped being updated from 2007, Lhasa moved to safeguard the data by providing ongoing access through a freely available interface; the Lhasa Carcinogenicity Database (LCDB)2. The LCDB was released in 2016 and has since been updated with additional data from the National Toxicology Program (NTP)3, increasing the data set to 7,745 studies covering 1,726 chemical substances. A recent update has also facilitated greater ease of access by enabling substructure and similarity-based structure queries.
TD50 values
As a measure of carcinogenic potency, the CPDB contained TD50 values, indicating the lifetime dose at which tumours would be observed in 50% of animals which would have remained tumour free at 0 dose. These have been reproduced for the LCDB4, which now displays both the Lhasa and CPDB TD50 values. This has enabled validation of the calculation method by comparison with the pre-existing CPDB values, as well as expansion of the data set by the calculation of new TD50s from the newly added NTP data.
Discrepancies between Lhasa and CPDB TD50 values
While there is a high correlation between the two sets of TD50 values, there are a number of instances where a CPDB TD50 is available but a Lhasa calculated value has not been supplied. There are several circumstances which may contribute to this:
- The CPDB used two calculation methods to produce TD50s; one using tumour incidence data from intercurrent mortality (lifetable method), and one using only the terminal sacrifice tumour incidence (summary method). When calculating the Lhasa TD50s, only the terminal sacrifice data was available (obtained from the CPDB site). Therefore, Lhasa TD50s have only been provided where the CPDB used the summary calculation method. While it would be possible to use the summary method to calculate TD50s for the remaining studies which originally used lifetable data, the resultant values are likely to be less accurate.
- Lhasa TD50s were not calculated for studies where the original author concluded treatment-related tumourigenesis was not observed.
- The CPDB assigned a notation based on the shape of the tumour incidence dose-response curve for each tumour. Lhasa TD50s were not calculated where this notation indicated the lack of a dose-response relationship.
- Lhasa TD50s were not calculated where only a single concentration of the test substance was used.
Case study- N-nitrosodimethylamine (NDMA)
NDMA is a member of the nitrosamine class of compounds which are of current interest due to their detection in samples of some marketed pharmaceuticals5. It is a potent carcinogen which has been well studied. However, there is a difference in the overall TD50s in the rat between the CPDB value (0.096 mg/kg/day) and the Lhasa value (0.177 mg/kg/day). To account for this, we must first understand how these per species values are generated. There may be multiple studies conducted for the same compound in the same species. The CPDB produced a per species TD50 by taking the most potent individual tumour TD50 from each study and calculating the harmonic mean; a process which has been reproduced using the Lhasa TD50s. However, as the Lhasa TD50s were not calculated for all tumours which had CPDB TD50s, there are fewer data points available when calculating the per species Lhasa TD50s.
Looking first at the tumours which have both a CPDB and Lhasa TD50, there is a high correlation between the two sets of values (Fig. 1). Species level TD50s derived from these values alone would be very similar.
Figure 1: Tumours with both Lhasa and CPDB TD50 values
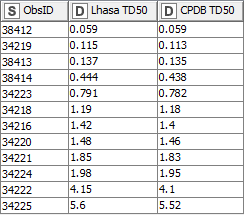
However, NDMA has additional tumours for which the CPDB TD50s are available but the LCDB TD50s are not. A number of tumours were observed in studies where only a single concentration of the test substance was used (Fig. 2). While this data was not considered appropriate for TD50 calculation by Lhasa, there are CPDB TD50s available, which will therefore contribute to the overall species TD50.
Figure 2: Tumours where only a single concentration was tested
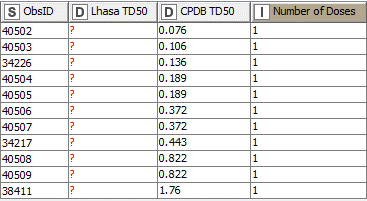 |
Finally, there are several tumours where the CPDB used the lifetable method to calculate TD50 values. While terminal sacrifice data was available for these, enabling the Lhasa summary method to be used, the two sets of values show a greater divergence (Fig. 3). The lifetable method consistently produced lower, more conservative, TD50s compared to those calculated using the summary method. As such, the summary method-based Lhasa TD50s were not included in the LCDB and so not used in determining the species level TD50s.
Figure 3: Tumours where CPDB used the Lifetable calculation method
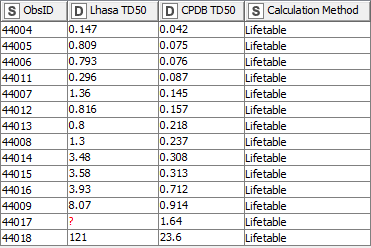 |
With the removal of inappropriate, or less accurate data points it is possible to see how the different summary TD50s ascribed to NDMA have come about. Both sets of values have been maintained in the LCDB to enable both continued access to the CPDB data and allow for transparency in how the data has been derived.
References
[1] Carcinogenic Potency Project (CPDB). https://toxnet.nlm.nih.gov/cpdb/
[2] Lhasa Limited. Carcinogenicity Database. https://carcdb.lhasalimited.org/
[3] National Toxicology Program. https://manticore.niehs.nih.gov/cebssearch/
[4] Thresher A., Gosling J.P., Williams R., Toxicology Research (2019) 8, 696-703. https://doi.org/10.1039/c9tx00118b
[5] European Medicines Agency (EMA), 2020. Nitrosamine Impurities in Human Medicinal Products. EMA/369136/2020. https://www.ema.europa.eu/en/documents/referral/nitrosamines-emea-h-a53-1490-assessment-report_en.pdf
Last Updated on January 25, 2024 by lhasalimited



