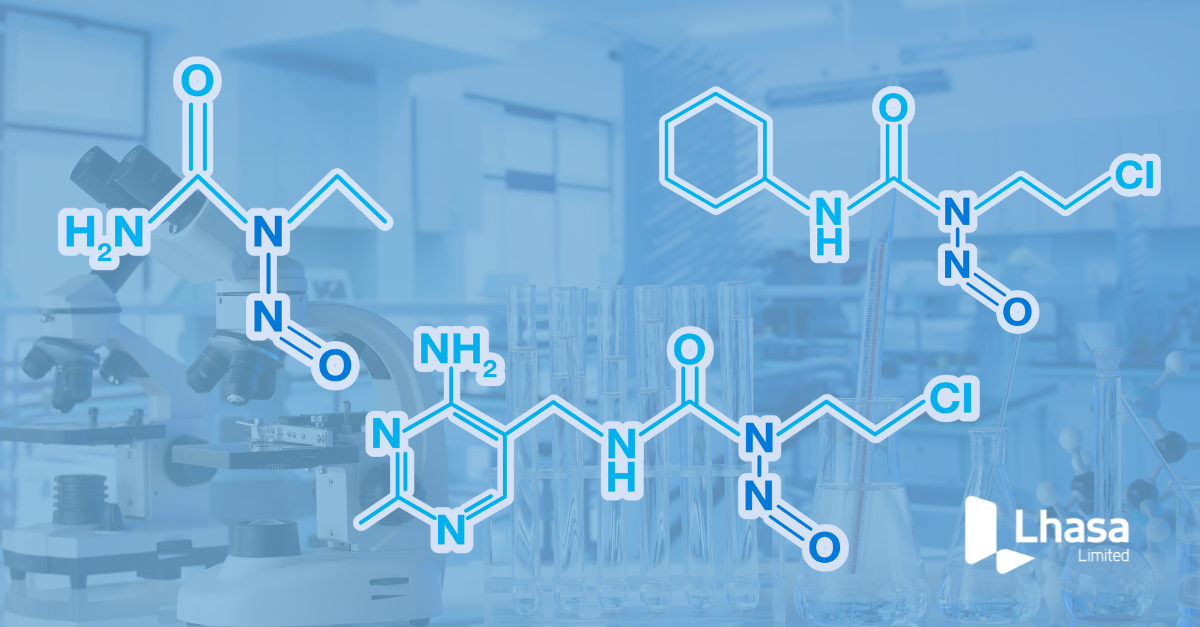
In a recent Lhasa Limited webinar, experts from BfR (the German Federal Institute for Risk Assessment), Syngenta, and Lhasa came together to …
The Science of Stability (SoS) 2025 conference, recently held in Philadelphia, brought together leading experts from the pharmaceutical industry and academia. Co-hosted by Lhasa Limited and …
The ICH M7(R2) Guideline (2023) continues to recommend the bacterial reverse mutation assay (Ames test), conducted in accordance with OECD 471, as …
In the ever-evolving landscape of pharmaceutical regulations, staying abreast of the latest guidelines is crucial for ensuring the safety and efficacy of …
A new paper, “Data-driven federated learning in drug discovery with knowledge distillation”, was recently published in Nature Machine Intelligence, the leading journal …
At Lhasa we know that there are many challenges involved in carrying out risk assessments for drug substances and drug products, to …