The ICH M7(R2) Guideline (2023) continues to recommend the bacterial reverse mutation assay (Ames test), conducted in accordance with OECD 471, as a primary tool for assessing the mutagenic potential of pharmaceutical impurities, including nitrosamines. While the Ames test is generally predictive of rodent carcinogenicity, concerns remain about its sensitivity under standard conditions, particularly when using 10% rat S9 for metabolic activation.
To address these concerns, regulatory agencies now encourage the use of enhanced Ames testing protocols. For background on this evolving guidance, read our earlier blog post: Current Status of the Ames Test for N-Nitrosamines.
A recent study by Bercu et al., published in Regulatory Toxicology and Pharmacology, presents significant progress in optimising the Ames test for nitrosamines. The paper details a multi-sector ring trial, conducted by the HESI Genetic Toxicology Technical Committee (GTTC), aimed at improving assay performance and interpretation.
What did the study involve?
The researchers evaluated 29 nitrosamines and 3 N-nitroso drug-like compounds, chosen to reflect a wide range of chemical structures and known carcinogenic outcomes. These were tested using five bacterial strains under a 30-minute pre-incubation protocol, designed to maximise metabolic activation and improve detection of mutagenic potential.
Key findings
- Increased sensitivity through optimised S9 conditions
The study revealed that using 30% hamster liver S9 in the Ames test significantly improved sensitivity for detecting mutagenic nitrosamines – reaching 90%. When 30% rat and 30% hamster S9 were used, the results from each were tested separately and then pooled to give the most conservative estimate, which increased sensitivity further to 93%. However, this improvement came at a cost to specificity, which dropped to around 45%, highlighting the trade-offs in test optimisation and the importance of choosing the right metabolic activation system.
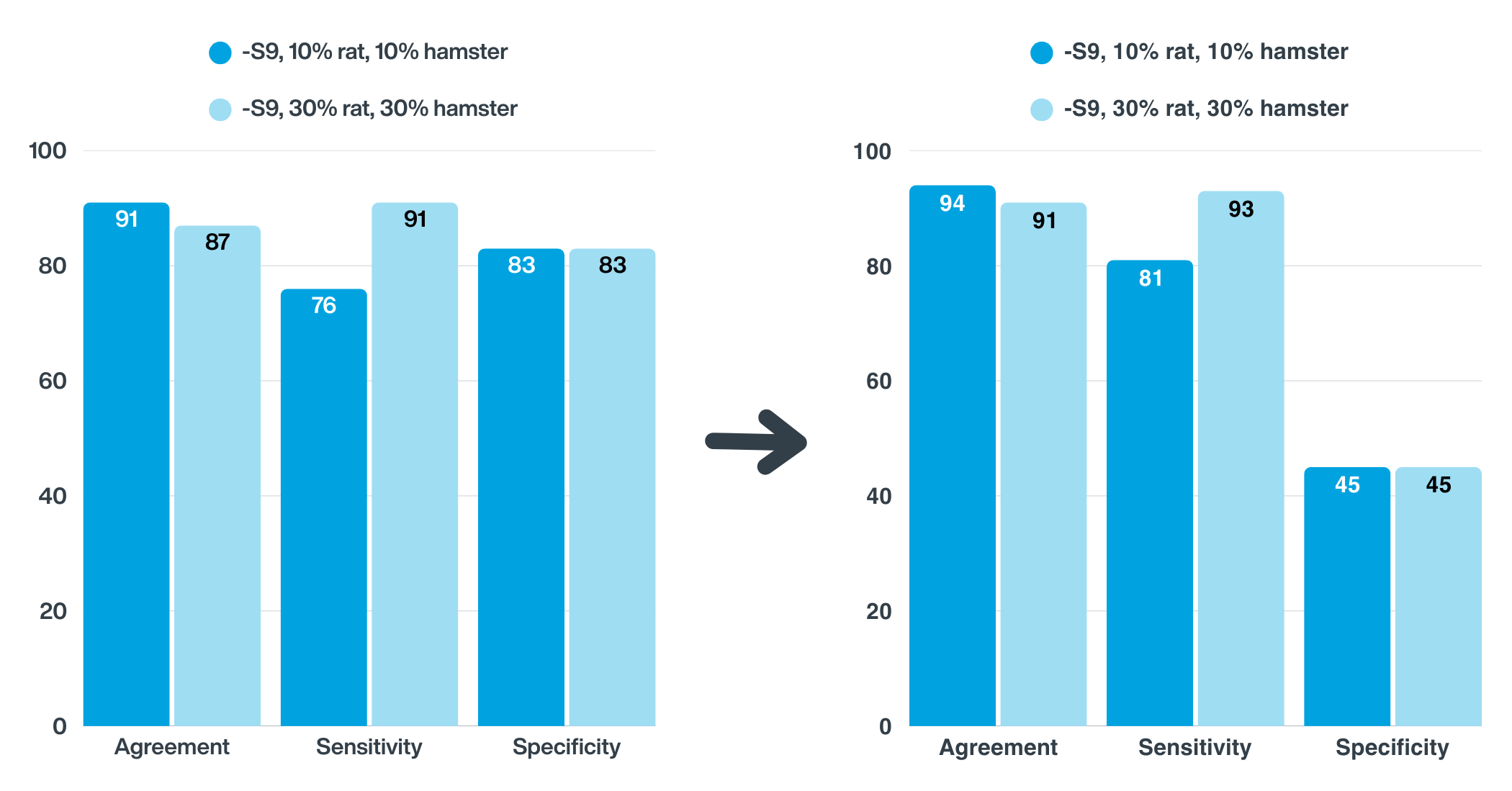
Fig. 1. Concordance between Ames test results and rodent carcinogenicity using different metabolic activation system (MAS) combinations. The presence of direct-acting nitrosamine mutagens decreased specificity, highlighting their influence on assay performance.
- Mechanistic insight reduces false positives
The researchers noted that excluding compounds which showed mutagenicity through mechanisms unrelated to direct DNA interaction, such as those testing positive without metabolic activation (-S9), improved the specificity of the test. This reinforces the importance of understanding the underlying mechanisms of action when interpreting Ames test results, particularly for regulatory applications.
For compounds that tested positive under metabolic activation conditions (+S9), Derek Nexus can support investigations into potential alternative mechanisms of action.
- Leveraging high-quality carcinogenicity data
Crucial to the study was the use of the Lhasa Carcinogenicity Database (LCDB). Each compound was assessed using Lhasa’s reliability scoring system, enabling confident classification of non-carcinogens and reducing uncertainty in test interpretation. This curated, high-quality data resource played a key role in ensuring robust, evidence-based conclusions, supporting both industry and regulators in their risk assessments.
Why this matters
This collaborative study demonstrates that carefully optimised assay conditions, supported by high-quality data and mechanistic insight, can significantly improve the predictive value of the Ames test for nitrosamines. Specifically:
- Greater sensitivity improves detection of mutagenic risks.
- Improved specificity reduces false positives, helping avoid unnecessary follow-up testing.
- Trusted databases like LCDB add confidence to the results, especially for negative findings.
Together, these advancements help ensure that the Ames test remains a robust and regulatory-relevant tool in the safety assessment of pharmaceutical impurities.
Discover the full study
Explore the full HESI GTTC publication on harmonised Ames testing of nitrosamines, offering high confidence in negative results and valuable insights for regulatory decision-making: HESI GTTC Ring Trial: Concordance between Ames and Rodent Carcinogenicity Outcomes for N-Nitrosamines (NAs) with Rat and Hamster Metabolic Conditions.
Interested in how Derek Nexus and Sarah Nexus can support your nitrosamine assessments? Get in touch or explore our solutions page.
Last Updated on May 19, 2025 by lhasalimited



