The ICH Q3B (Impurities in New Drug Products) guideline provides guidance on the qualification of impurities in new drug substances produced by chemical syntheses. This article discusses how Zeneth, Lhasa Limited’s expert decision support software for predicting the forced degradation of organic compounds, can help to satisfy ICH Q3B.
Scope of the ICH Q3B guideline
The second revision (R2 – July 2006) of the ICH Q3B guideline is more consistent with two other relevant ICH guidance’s; Q3A(R): Impurities in New Drug Substances and Q3C Impurities: Residual Solvents.
The guideline addresses impurities that are found in a new drug product, specifically those that are degradation products of the drug substance (Active Pharmaceutical Ingredient (API)) or reaction product(s) of the drug substance with an excipient or the immediate container/packaging.
True impurities (non-degradation type) that are present in the excipient or in the new drug product or impurities that have been extracted or leached from the container packaging are not covered by this guidance. However, a rationale should be provided for these types of impurities if they have been detected e.g. they could be processed impurities from the drug substance or impurities from excipients.
The guidance does not apply to biological/biotechnological products, peptides, oligonucleotides, radiopharmaceuticals, fermentation products and semisynthetic products derived therefrom, herbal products, and crude products of animal or plant origin.
– Thresholds for identification of degradation products
The guidance specifies that for the new drug product attempts should be made to include a list of all potential degradation products expected to occur during the manufacture of the commercial product and under recommended storage conditions. In addition, stability studies, knowledge of degradation pathways, product development studies and laboratory studies should be used to characterise the degradation profile. The degradation products observed in stability studies should be identified when present at levels greater than the identification threshold. Degradation products which are less than or equal to the threshold do not need to be identified (see table 1 below).
The thresholds for degradation products are expressed either as a percentage of the drug substance or as total daily intake (TDI) of the degradation product. Lower thresholds can be appropriate if the degradation product is unusually toxic, however higher thresholds should be scientifically justified.
| Maximum daily dose (mg) | Threshold |
| < 1 | 1.0% or 5 µg TDI, whichever is lower |
| 1 – 10 | 0.5% or 20 µg TDI, whichever is lower |
| 10 – 2000 | 0.2% or 2 mg TDI, whichever is lower |
| > 2000 | 0.1% |
Table 1: thresholds for degradation products (in new drug product).
– Decision tree
The decision tree for the identification and qualification of a degradation product (illustrated in Figure 1) describes considerations for the qualification of degradation products when thresholds are exceeded.
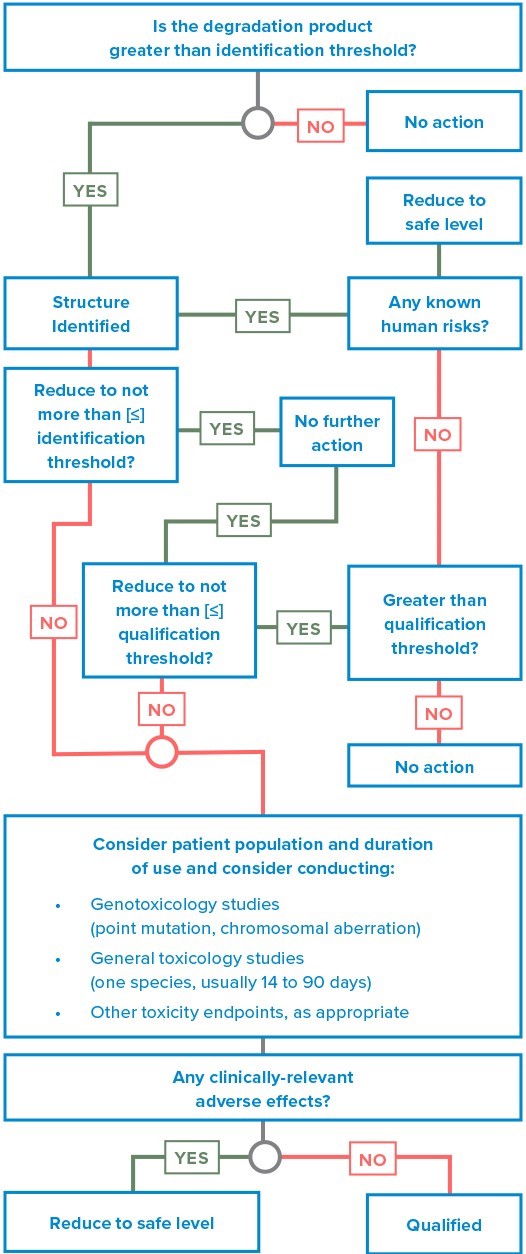
Figure 1*: ICH decision tree for identification and qualification of a degradation product.
* adapted from https://www.eag.com/resources/whitepapers/detecting-mutagenic-impurities/
How can Zeneth help to satisfy ICH Q3B?
Zeneth is an expert Knowledge-based system for the prediction of forced degradation. It can simulate the environmental conditions that are typically used in stability studies to generate a prediction for a particular drug compound. There are two core components to Zeneth.
The first is the encapsulation of known degradation knowledge within its knowledge manager. This houses all the transformations (with references) and excipients. The chemistry within each of the transformations has been compiled from specialist chemistry books, published journal articles as well as from proprietary data via data sharing initiatives. The knowledge manager therefore contains vast amounts of knowledge of degradation pathways. Each individual transformation includes information about the environmental conditions that are required to invoke the pathway (Figure 2).

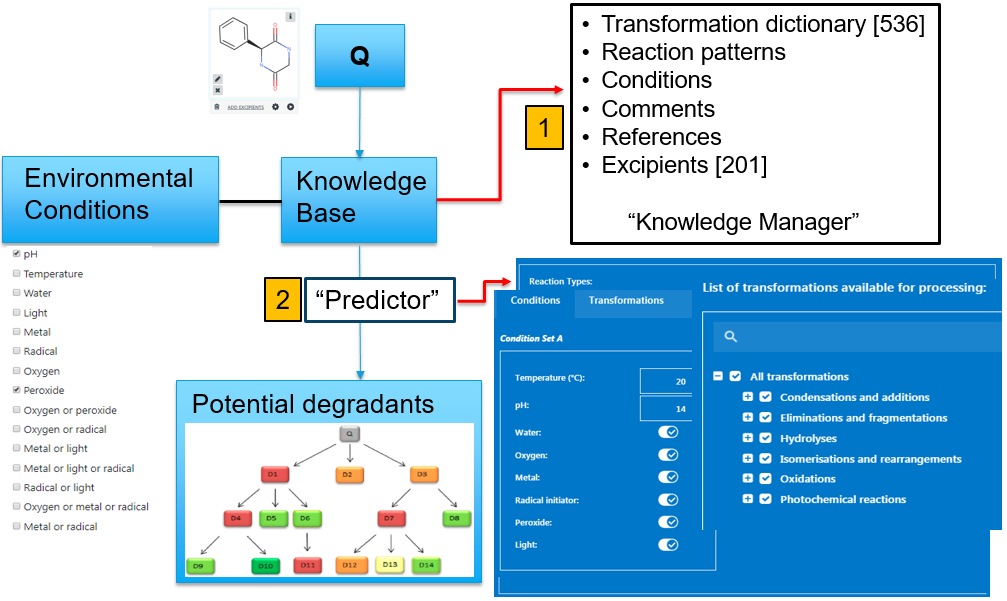
Figure 2: overview of a Zeneth degradation prediction.
The second component is the predictor. Here the query compound (usually the API) is specified together with the environmental conditions. In addition excipients (or other impurities) and their common degradants can be run alongside the API to assess the potential of intermolecular reactions.
As illustrated in Figure 3, benzaldehyde and benzoic acid are known degradants of the excipient benzyl alcohol and therefore can be optionally selected alongside the main excipient.

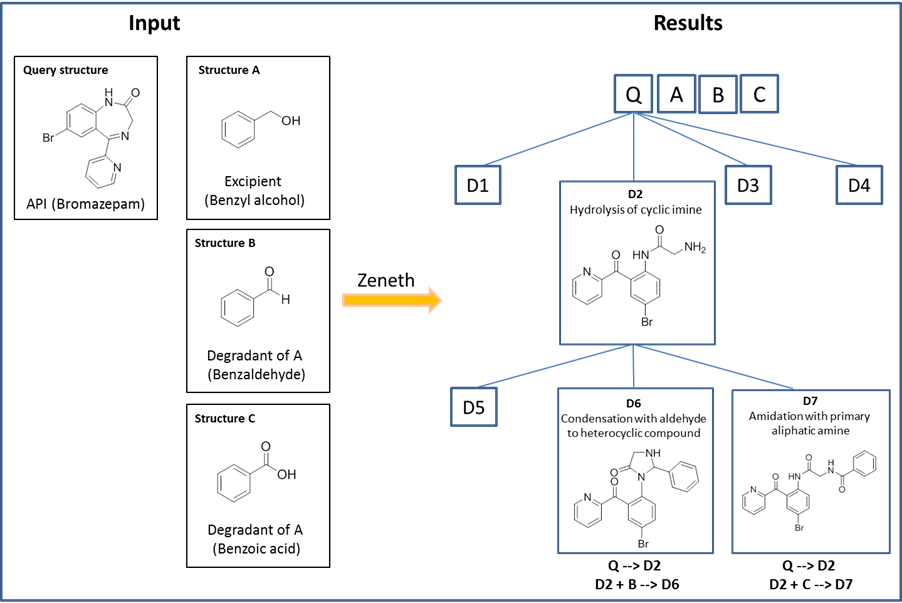
Figure 3: Reaction of excipients with an API.
The predictor generates a set of potential degradants by matching the key functional groups of the query molecule with the chemistry held within each of the transformations. Each time a functional group on the query molecule produces a match with a transformation (and its environmental conditions), then it is triggered and the products of the transformation are generated as degradants. Some transformations are more likely than others and Zeneth uses a reasoning engine to score each degradant as it appears in the results tree (Figure 4).
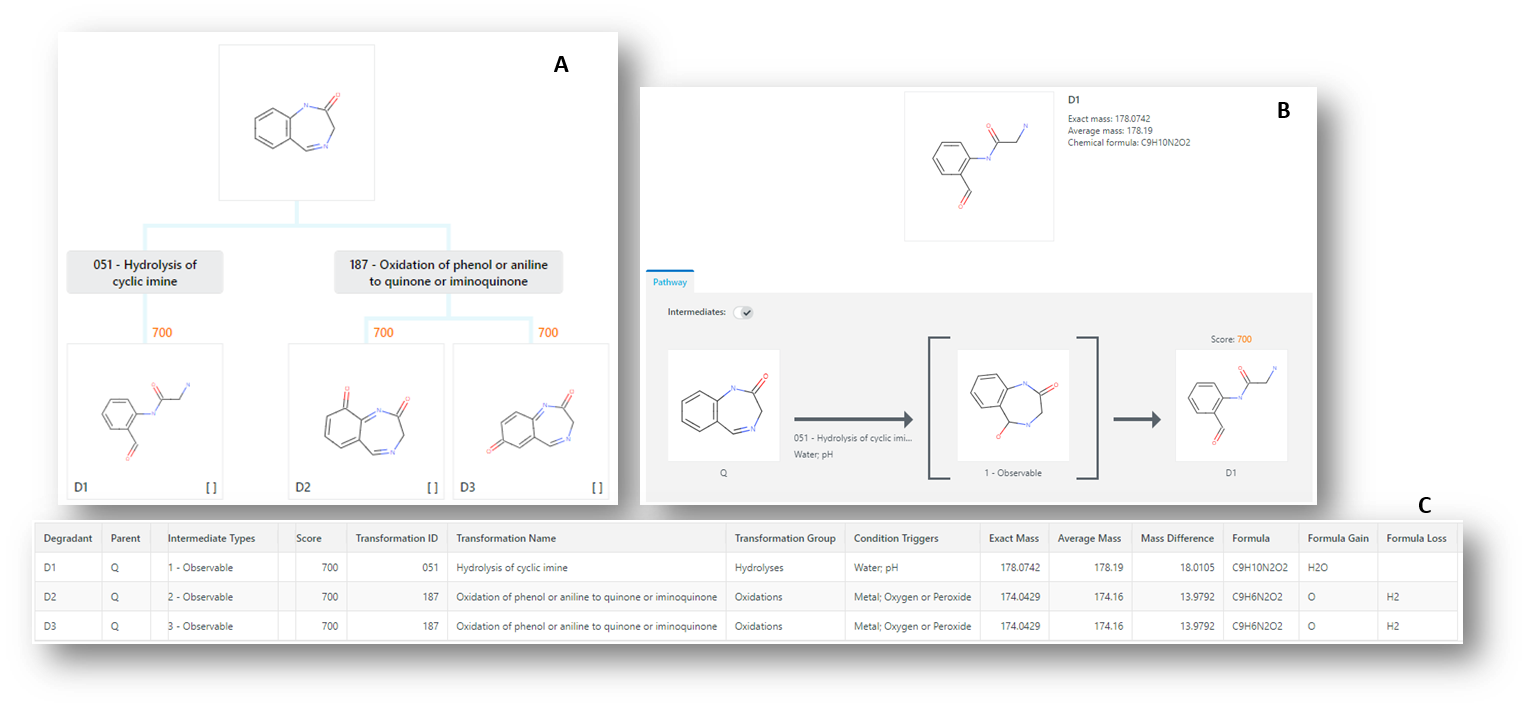
Figure 4: Zeneth results; (A) degradation tree, showing all potential degradants, (B) transformation pathway showing intermediate (using knowledge of degradation pathways), (C) table of results with mass spec data.
The main emphasis of the ICH Q3B guidance is placed on detecting and identifying the degradation products of the drug substance (API) – either on its own or if excipients are present its reaction with them or with impurities of the immediate container/packaging.
As discussed above Zeneth can predict the forced degradation pathways of organic compounds under various environmental conditions. This in silico tool can therefore support the drug development process by being able to run excipients and counterions alongside the drug substance to assess the potential for intermolecular reactions. Zeneth can be used in conjunction with analytical data to help elucidate the structure of products generated during forced degradation studies and has been designed primarily to aid the interpretation of experimental data thus helping to make informed decisions about the final drug product.
Definition of terms used in the ICH 3QB guidance
Degradation Product: an impurity resulting from a chemical change in the drug substance brought about during manufacture and/or storage of the new drug product by the effect of, for example, light, temperature, pH, water, or by reaction with an excipient and/or the immediate container closure system.
New Drug Substance: the designated therapeutic moiety that has not been previously registered in a region or member state (also referred to as a new molecular entity or new chemical entity).
Degradation Profile: a description of the degradation products observed in the drug substance or drug product.
Identified Degradation Product: a degradation product for which a structural characterisation has been achieved.
Impurity: any component of the new drug product that is not the drug substance or an excipient in the drug product.
Specified Degradation Product: a degradation product that is individually listed and limited with a specific acceptance criterion in the new drug product specification. A specified degradation product can be either identified or unidentified
Unidentified Degradation Product: a degradation product for which a structural characterization has not been achieved and that is defined solely by qualitative analytical properties (e.g., chromatographic retention time).
For more information about Zeneth, visit our impurity and degradant control page. Get in touch with the Sales Team for a free demonstration.



